Study of High-Mannose Glycan Truncation on Von Willebrand Factor (VWF)
Abstract
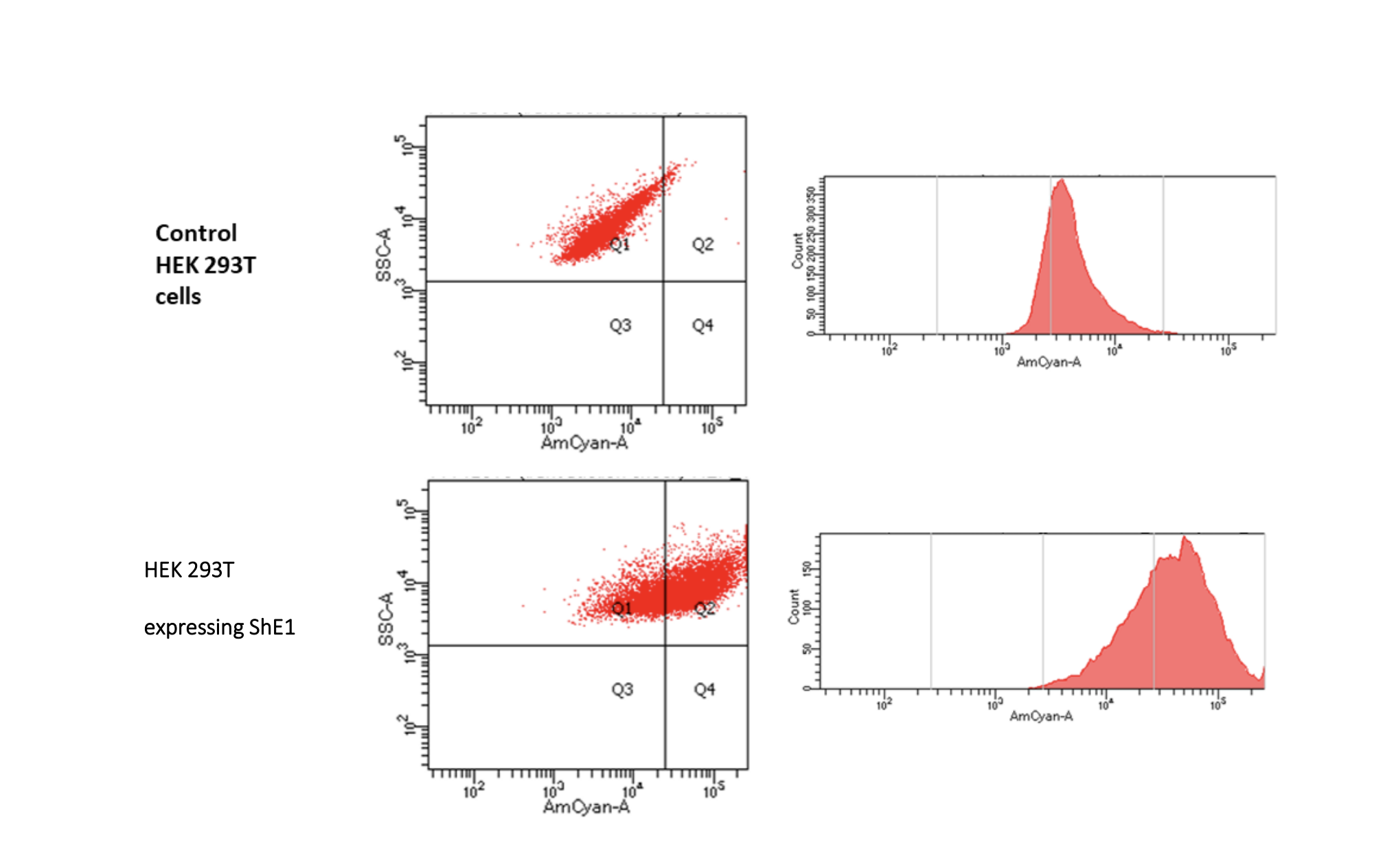
Von Willebrand factor is a large multimeric plasma glycoprotein that plays a key role in maintaining hemostasis and thrombosis. Quantitative and functional deficiency in VWF, majorly inherited, lead to a condition called von Willebrand disease (VWD). Recent advances in biopharmaceuticals has paved way for the supplementation of recombinant VWF to treat VWD. VWF is heavily glycosylated and makes it vital to study the role of these glycans in protein function and half-life. Previous studies have shown that reducing high-mannose N-glycans by genetic methods or using chemical inhibitors increases VWF half-life circulation. In this study, we biologically expressed endoglycosidase H (EndoH) both transiently and stably within mammal cells while simultaneously producing recombinant VWF in them. Endo H truncates high-mannose N-glycans on glycoproteins at the chitobiose core leaving behind a GlcNAc attached to the protein at Asn. We want to ensure that the structural property of VWF is not disturbed by truncation of high-mannose glycans on its surface. Also, the lectins on the stably expressing EndoH (ShE1) cell line were studied to understand the EndoH protein activity.